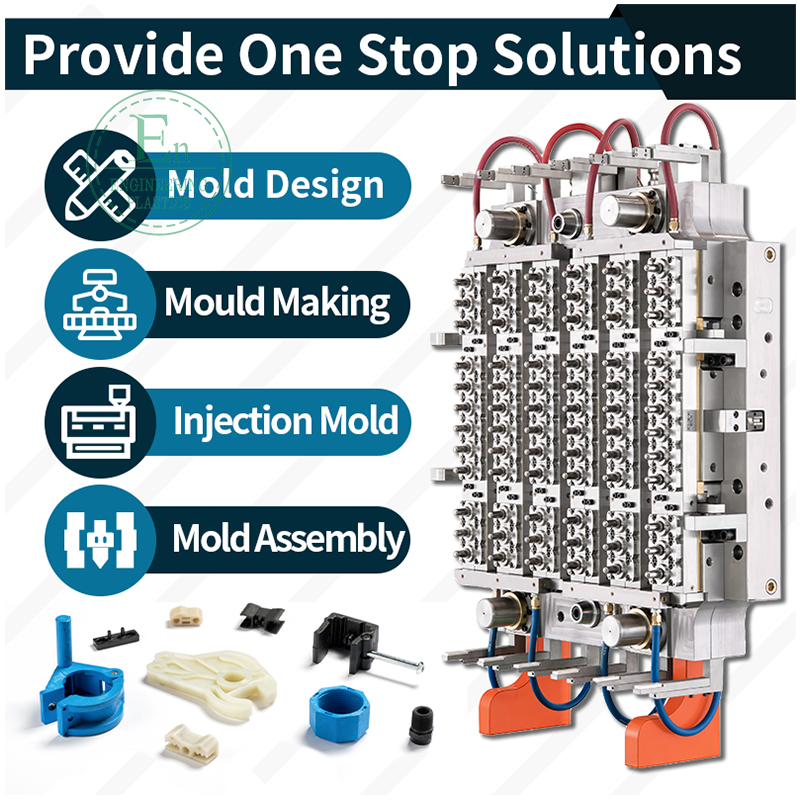
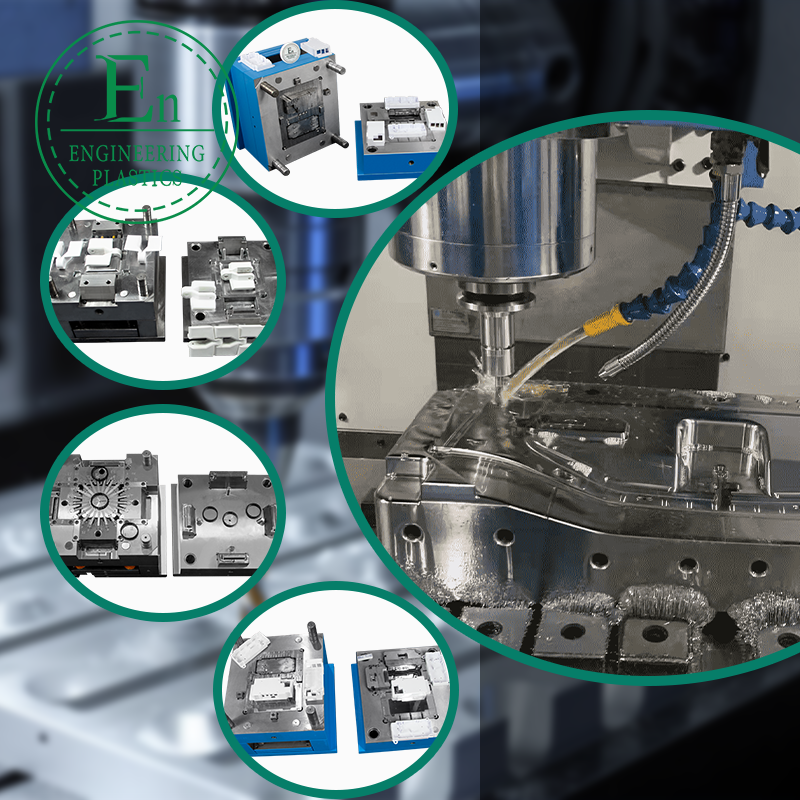
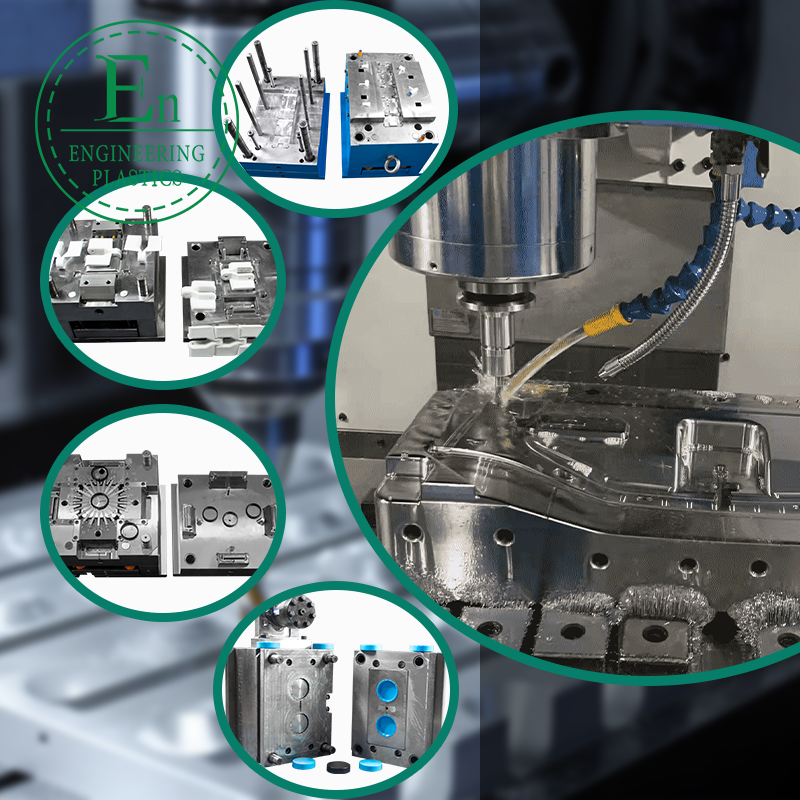
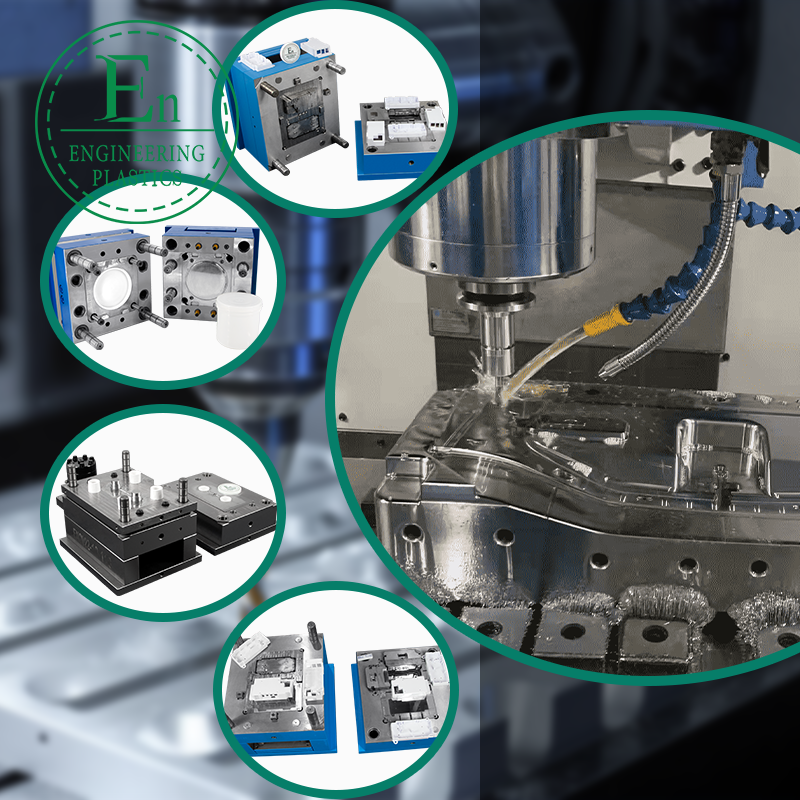
Medical Injection Molding: A Complete Guide for Global Medical Device Companies
Medical device teams do not buy plastic parts; they buy reliability. In regulated markets like the EU and the United States, a small molding defect can cascade into regulatory delays, field failures, recalls, and brand damage. That is why medical injection molding is less about “making parts” and more about building a controlled manufacturing system: validated tooling, stable processes, traceable materials, and documented change control.
This guide explains what medical injection molding is, which device categories it supports, what quality and compliance expectations buyers should demand, and how to qualify a supplier for Europe, the U.S., and Russia. If you are planning a new program or transferring an existing mold, you can use the checklists and examples below to reduce risk and shorten your time to market.
What Medical Injection Molding Means in Practice
Medical injection molding is the production of plastic components for medical devices using injection molding machines and engineered tooling, under a quality system designed for patient safety and regulatory compliance. Compared with general injection molding, medical programs typically require tighter dimensional control, stricter documentation, and more robust validation.
In many projects, the “molding” scope includes more than the molded part. It can include tool design (DFM), moldflow analysis, steel selection, surface finishing, cavity balance for multi-cavity tools, controlled material handling, cleanroom production, assembly, packaging, and full verification/validation support.
Typical Medical Device Applications for Injection Molded Parts
Injection molded components are used across nearly every segment of healthcare. Common categories include:
- Drug delivery: inhalers, auto-injector housings, pen injectors, dose counters, caps and safety features
- Diagnostics and IVD: cartridge bodies, optical housings, sample cups, analyzer enclosures, fluidic manifolds
- Surgical and procedural: instrument handles, knobs, protective covers, disposable tool components
- Patient care and disposables: connectors, clamps, filter housings, syringe components, tubing interfaces
- Medical electronics: wearable housings, battery doors, sensor shells, docking components
These products often combine functional requirements (leak tightness, snap fits, fatigue resistance) with strict cosmetic expectations (no flow marks in optical areas, consistent color, controlled gloss) and hygienic considerations (smooth, cleanable surfaces).
Regulatory and Quality Expectations Buyers Must Align On
Quality Management System and Documentation
A medical injection molding supplier should be able to operate under a disciplined quality management system. Many buyers prefer suppliers aligned with ISO 13485 and ISO 9001, and who can support documentation needs such as:
- Device history records and traceability (lot/batch, resin certificates, process parameters)
- Incoming inspection, in-process controls, and final inspection records
- Calibration and preventive maintenance logs
- Nonconformance handling (NCR), CAPA, and root-cause analysis
- Change control and revision management for tooling and process
Even if the molded component is not the final device, your supplier’s records and controls can become part of your technical file or regulatory audit trail.
Validation: IQ, OQ, PQ and Process Capability
For medical programs, validation is often as important as machining. A typical validation pathway includes: IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification). Buyers may also request process capability evidence such as Cp/Cpk studies on critical dimensions, gage R&R for measurement systems, and control plans that define sampling frequency and reaction plans when trends shift.
Cleanroom and Contamination Control
Not every medical part needs a cleanroom, but contamination control always matters. If your device contacts the patient, drug product, or sterile pathway, cleanroom molding and controlled handling become more important. Buyers should define required cleanroom class, material and operator flow, cleaning and line clearance procedures, and packaging method.
Key Engineering Requirements for High-Precision Medical Molding
Tolerance, Repeatability, and Tooling Fundamentals
Medical projects often specify tight tolerances for sealing surfaces, snap-fit engagement, optical alignment, or mating to metal components. Precision is not just about cutting steel; it is also about maintaining repeatability over thousands or millions of shots. Look for robust mold design, optimized cooling, correct steel selection, wear-resistant inserts, and cavity balance for multi-cavity tools.
Material Selection: Medical-Grade Plastics and What They Enable
Resin choice impacts performance, compliance, and cost. Common medical-grade materials used in injection molding include PP, PC, ABS or PC/ABS, POM (confirm suitability), TPU, and high-performance polymers like PEEK. Your supplier should guide you on shrinkage behavior, moisture sensitivity, and how sterilization methods (ETO, gamma, steam) can affect properties and dimensions.
Surface Finish, Cosmetic Control, and Function
Mold surface finishing choices can influence cleanability, gloss/matte appearance, optical clarity, and release. Define cosmetic standards clearly and confirm how the supplier prevents sink, splay, burn marks, flash, and weld lines in critical areas.
Design for Manufacturability (DFM) and Moldflow: What Buyers Should Expect
A strong DFM review should happen before cutting steel, covering draft, wall transitions, ribs/bosses, gating/venting, ejection, tolerance capability, sealing features, assembly sequence, and inspection strategy. Moldflow analysis is valuable for thin-wall stability, multi-cavity balance, weld line control, air trap reduction, and warpage prediction.
From Prototype to Production: A Practical Program Roadmap
Phase 1: Prototype and Feasibility
Many buyers start with rapid tooling or single-cavity prototype molds to validate geometry and assembly. Deliverables often include first-article samples, dimensional reports, and parameter recommendations.
Phase 2: Bridge Tooling and Pilot Builds
Bridge tooling supports pilot builds, verification activities, and packaging/labeling validation. If the design will move to a high-cavity mold later, bridge tooling should mimic final gating and shrink behavior when possible.
Phase 3: Production Tooling, Validation, and Scale
Production molds are built for longevity, repeatability, and throughput. This phase typically includes acceptance criteria, sampling plans, IQ/OQ/PQ reporting, control plans, capability monitoring, and spare parts strategy.
How to Evaluate and Qualify a Medical Injection Mold Supplier
Commercial and Project Management Fit
Look for clear RFQ expectations, transparent lead times, a structured sampling plan, communication cadence, escalation path, and disciplined engineering change handling.
Manufacturing Capability Checklist
Request evidence of precision machining/EDM capacity, metrology (CMM/optical), parameter control, preventive maintenance, controlled resin handling, and traceability systems.
Risk Management and Business Continuity
Ask about redundancy for critical machines, spare inserts/cavities for high-volume tools, contingency planning, and tool-transfer support.
Cost Drivers, Lead Times, and How to Avoid Hidden Costs
Key drivers include tolerance risk, cavity count, hot runner complexity, surface finish, resin abrasiveness, validation workload, and tool life expectations. Avoid surprises by aligning on acceptance criteria, measurement method, sample size, and the definition of “production-ready” early.
Mini Case Example: Diagnostic Cartridge Tooling for the EU Market
A European diagnostics company needed a thin-wall cartridge body with controlled weld line placement and stable fit to an optical module. We approached it with DFM + moldflow, optimized cooling, a controlled process window, and a CTQ measurement plan—reducing iterations, stabilizing mass production, and supporting documentation for the customer’s technical file.
Why Work with Guangdong Engineering Plastics Industrial Group Co., Ltd.
Guangdong Engineering Plastics Industrial Group Co., Ltd. provides injection molding and customized mold manufacturing services for international B2B customers. For medical device programs, we focus on precision tooling, stable process control, and practical documentation support so buyers can reduce risk and move faster from development to production.
- Engineering-first collaboration: DFM feedback, manufacturability optimization, risk review before tooling
- High-precision mold manufacturing capability for demanding tolerance requirements
- Export-focused support for Europe, the U.S., Russia, and global programs
- Responsive project management and clear cross-border communication
Request a Quote: Information to Send for a Fast, Accurate RFQ
- 2D drawings and/or 3D files (STEP preferred)
- Material requirement (or application + sterilization method)
- Annual volume forecast and target cavity count (if known)
- Critical-to-quality dimensions (CTQs) and inspection expectations
- Target markets (EU, USA, Russia) and compliance needs
- Packaging preference and any assembly requirements
Conclusion and Call to Action
Medical injection molding succeeds when tooling, process, and quality systems work together. The right supplier helps you design for manufacturability, validate the process, and maintain stable output through scale-up and ongoing production.